COVID
A year and a half into the pandemic, and with the benefit of 20/20 hindsight, I will gladly admit that we fucked up badly by culling our mouse colonies back to a fifth of their normal size in March of 2020 – it’s taken us over a year to get everything back up to size and running again, and meanwhile everyone else just #DGAF and carried along doing mouse work. Ugh!
Still, I get to take out my rage by shouting obscenities at the moronic antivax protesters outside the hospital on my daily bike ride. I should really invest in a Super Soaker filled with MMTS to spray at them.
PAPERS
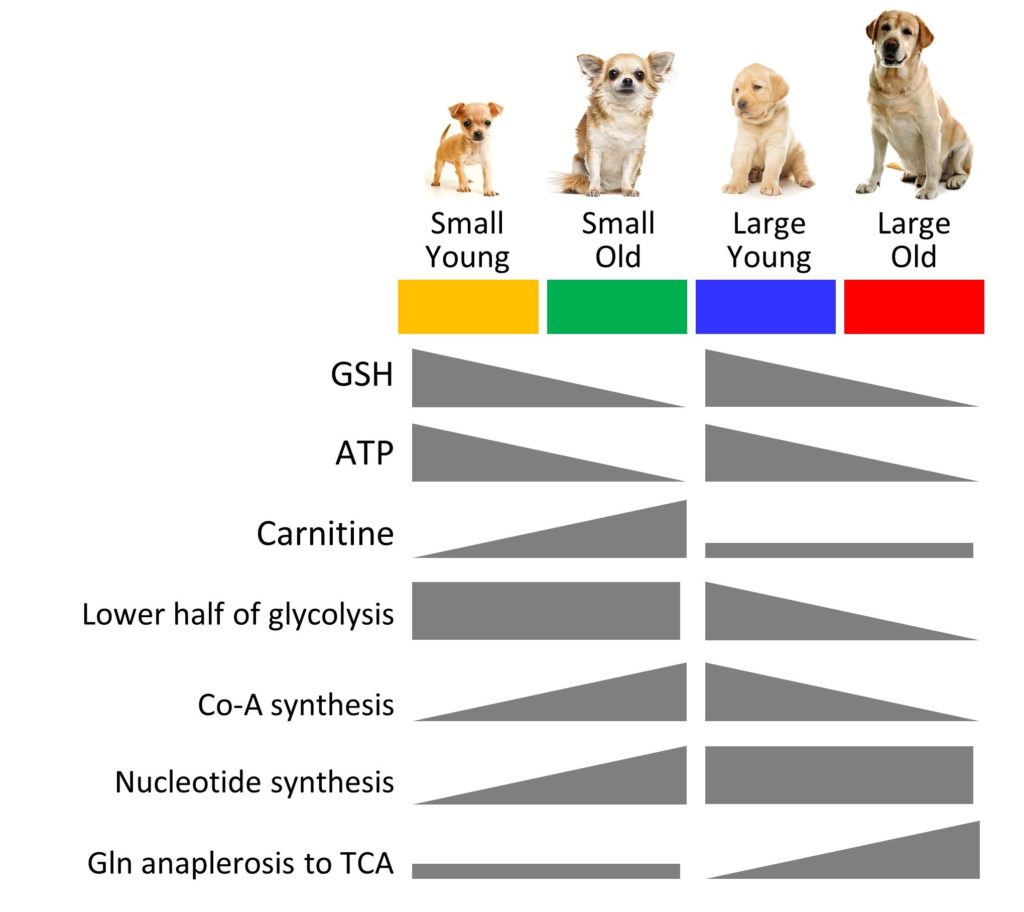
(1) Our paper with Ana Jimenez of Colgate University, on metabolomics of young and old small and large breed dogs, was finally published in Geroscience. This was part of a larger study by Ana, aimed at addressing the question of why large dogs die prematurely (which is opposite to most of the rest of biology, where large animals live longer). The graphical summary below highlights some key differences in metabolite abundances between fibroblasts from the 4 different groups. Of particular interest (to me) is that carnitine seems to go up as small dogs age, but not large dogs, which might suggest some differences in ability to burn fat, which could be targeted pharmacologically in older dogs.
(3) Our paper on a modified blue-native (BN) gel method was also published, as part of the Methods in Molecular Biology book series. Blue-native is a method for looking at large protein complexes, including so-called “supercomplexes” (SCs) of the mitochondrial respiratory chain. There’s been a lot of debate about the functional importance of SCs, and this paper is the result of a project to streamline the method to reduce a potential artifact source. The project itself was done by an undergrad summer intern, Megan Ngai, who is going to be starting medical school at SUNY upstate next year.
The premise of the modified method is quite simple… most BN methods involve treating your samples somehow, then extracting with detergent, and this step necessarily involves pelleting the mitochondria by centrifugation, prior to extraction. The problem is, doing so might select for “good” or dense mitochondria, and many of the perturbations that have been shown to alter SC formation are also associated with alteration of mito’ density (e.g. respiratory state, PT pore opening). So, what we did was to develop a compromise mito’ buffer that is suitable for both mito’ incubations/treatments and for extraction. This eliminates the pelleting step, so the mito’s that get put on the gel are not “sampled” in any way – they’re representative of the whole population. The upshot is that we find many perturbations of mito’ function simply do not pan out with differences in SC formation or abundance. That’s not to say all the data so far on SC’s is artifactual, but it does suggest that acute alterations in mito’ function really don’t seem to be linked to SCs in a functional manner.
FUNDING
The main R01 that funds the lab (HL-071158) is sadly now in no-cost extension, as the competing renewal application was reviewed in February but missed the payline by 2%. A revision was submitted in July and will be reviewed in October, so we are currently awaiting with fingers crossed. If the news is bad again, it will be the end of the line for this grant which my lab has held since 2003.
Our other funding is in the form of an R56 1 year award (at half modular budget) to gain more data for Aim 2 of the parent R01 application (DK-126659). That award is also in NCE, since the high-fat-diet studies that underpin it were delayed due to the mouse problems outlined above.
So, we’re running on fumes for the time being, hoping for a funding hit and trying to remain productive and optimistic in the interim. This cyclical nature of funding is nothing new, but it bites really hard when a grant you’ve had since your lab began (and which has produced over 100 papers in 17 years) might not make it.
PROJECT HAPPENINGS
- We have successfully established a breeding colony of Glo1-/- (glyoxalase-I knockout) mice. The founders came from Jim Galligan in Arizona, and we’re using them to explore methylglyoxal stress in the Alkbh7-/- mice, as reported here.
- On a related note, we tried last year to get a “matters arising” published in Nature, regarding a paper on lactylation of lysine residues. A pre-print of our letter is here, in which we point out 3 things. First, the MW of the lactyl-lysine moiety is the same as that of MGO-induced carboxyethyllysine, so mass spec’ alone cannot distinguish these PTMs. Second, the anti-lactyl-lysine antibody developed by the authors (which they sell through the PI’s company) appears to recognize CEL (or another MGO-induced adduct) better than it recognizes lactyl-lysine. Thirdly, the concentrations of lactate used to induce lactylation (25mM) are inhibitory to the enzyme GLO-2, such that they will elevate S-lactoyl-glutathione levels, and this is probably the underlying mechanism driving lactylation (as has been shown by Galligan). Unfortunately, after a year of dicking around Nature decided to reject our letter (and the author’s response) and the authors were quite rude to us about the whole affair, accusing us of stealing their ideas, and telling us to stop confusing people. All highly unprofessional, but par-for-the-course in glam-publishing land. Ugh!
- A paper on ERK5 signaling and cardiac mitochondrial hibernation with Jun-Ichi Abe should be out in Redox Biology soon.
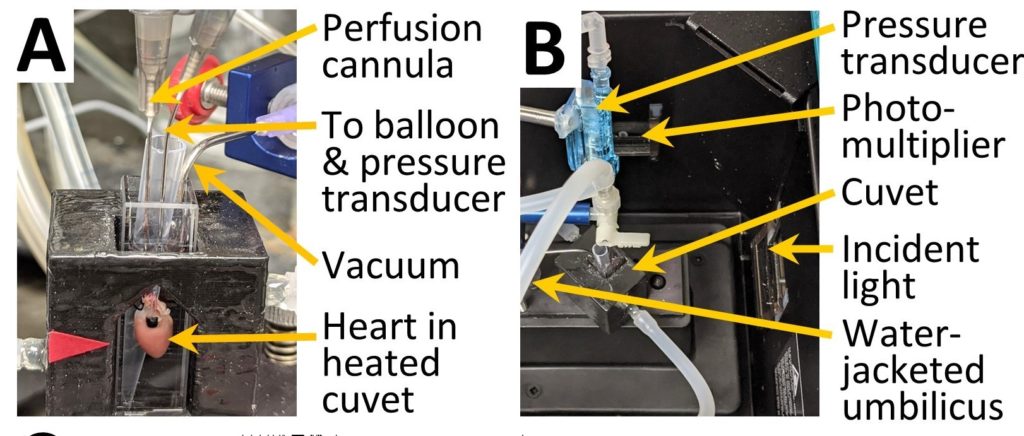
- Grad student’ Alex Milliken’s project on measuring ROS in intact beating mouse hearts is progressing rapidly. We have built a Langendorff perfusion apparatus into a spectrofluorimeter, which allows measurement of reflectance-fluorescence of the heart, as shown in the picture below:
- Using this system we can measure not just parameters like ROS, but also NADH, FADH2, membrane potential (TMRE) and mito’ PT pore opening. While others have done this in larger hearts (guinea pig or rat) this is the first such system in mouse heart, and also the only system with simultaneous measurement of cardiac function at the same time, with a pressure transducer balloon. Thus, we can relate changes in fluorescence to changes in cardiac function in real time.
- We’re still working on ALKBH7, trying to figure out its biological function. In that regard, we were intrigued by a recent paper that claims to have discovered a role for the enzyme in regulating mitochondrial ribosomal and RNAs. To us, this is weird, because previously the crystal structure of the enzyme was specifically called out for lacking any binding sites for nucleic acids, as seen in some other ALKBH family members! The authors of the new paper seem to gloss over this by simply stating the enzyme has binding loops conserved from E. coli AlkB. But we’re not convinced the diagrams show any such conservation (rather, just a bunch of disordered structure). Adding to the complication, nothing about this new finding has any impact whatsoever on the underlying phenotype of the Alkbh7 knockout – namely, they don’t die of necrosis! Why would knocking out an RNA demethylase have any impact on acute necrotic signaling? So, this doesn’t really change much about our ongoing quest to determine how the enzyme is involved in necrosis, and how it interfaces with glyoxal metabolism (which the authors conveniently fail to mention, or cite our work). The proteomic data from the paper (which mainly uses siRNA knock down) also disagree with our own findins in knocout mouse hearts – namely they report large scale reductions in mitochondrial transcripts, but we saw no differences in any of the respiratory chain complexes, and no differences in mitochondrial enzyme activities. It could be this is highly cell-type specific, with ALKBH7 having an RNA-processing role in dividing/proliferating cells, versus more of a metabolism/cell-death role in terminally differentiated tissues such as the heart. Lots still to learn about this fascinating protein!
TRAVEL & CONFERENCES
We attended the AHA BVCS conference, where Alex presented a poster. We will also be at the Society for Heart Vascular Metabolism (SHVM) conference in later September, and the Society for Redox Biology & Medicine (SfRBM) conference in November. The latter is supposed to be in person, with Paul presenting at the sunrise free radical school, but I’d say with Covid on the rise, the likelihood of us going to Savannah GA any time soon is not looking high.
Hopes for in-person travel are slightly better however, for an upcoming Keystone Meeting on Mitochondria, Metabolism & Heart, in Breckenridge Colorado next February. Fingers crossed for less moronic behavior by the antivax population between now and the end of the year!