I was at a meeting yesterday discussing cancer treatments, and the use of oxaloacetate (OAA) as an anti-cancer drug came up. This prompted some more reading, and my conclusion is there’s a lot of craaaazy stuff out there, with very little actual evidence for pushing this molecule into the clinic.
Background on OAA
First, for the uninitiated, OAA is one of the metabolites in the Krebs’ tricarboxylic acid (TCA) cycle in mitochondria. OAA is made by malate dehydrogenase (MDH)…
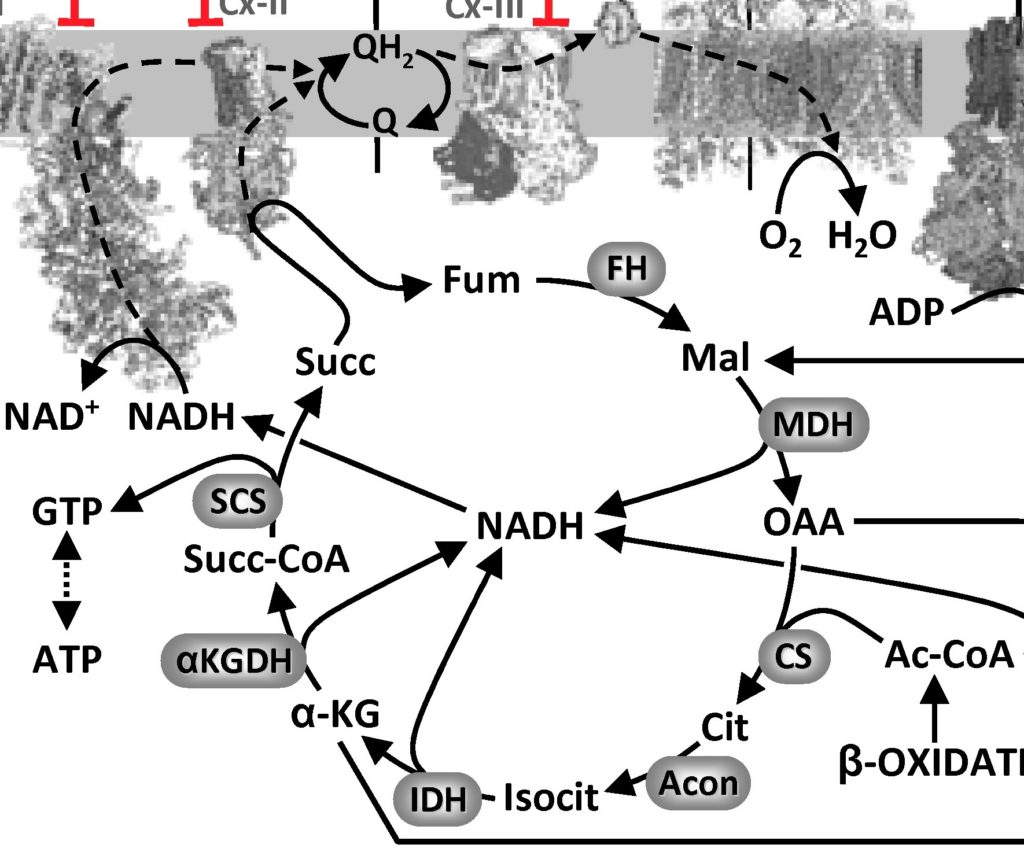
The MDH reaction is energetically unfavorable (ΔG +29.7 kJ/mol), but in reality that’s overcome by the next reaction in the Krebs’ cycle (citrate synthase, CS) being very favorable (ΔG -31.5 kJ/mol) so OAA gets removed as soon as it is made and that pulls the cycle forward.
OAA as a Drug?
The development of OAA from metabolite to drug appears to be pushed mostly by a company called “Terra Biological“. They’re selling OAA as a nutraceutical dietary supplement (only $49 for a month’s supply!) with all of the usual snake-oil claims that accompany such operations (“boosts energy”, “enhances mental focus”, blah-di-blah). Oh, and it allegedly mimics caloric restriction (CR) so will help you live longer, so there’s that. Here‘s a slide deck by the CEO of the company, Alan B. Cash, pushing all kinds of uses of OAA, from cancer to Parkinson’s and Alzhemier’s disease and everything in between (and here‘s another link in case that one goes down). The company received a warning letter from the FDA in 2017, for making drug-like claims about OAA supplements.
Despite this craziness, the company is now pushing OAA into the disease area of Glioma, by promoting “Cronoxal” (aka OAA) as a “medical food” for glioma patients. This DropBox link contains a bunch of hokey information from the company on the alleged benefits. For a time, Cronoxal was also known as “Gliaxal”. It’s also marketed under the name Benagene a “genomic aging supplement” (whatever that means) and as Jubilance for PMS relief. Cash also runs MetVital, yet another company developing OAA in a bunch of disease areas.
So where’s the evidence?
As is often the case with the murky waters of poorly-regulated dietary supplements are a number of shortcomings with the actual underlying scientific evidence for OAA’s effects. Most of the hype appears to hinge on this 2009 paper in Aging Cell, claiming that addition of 8 mM (!) OAA to growth media enhances lifespan in the nematode C. elegans. A notable feature of this paper is that it was published in September 2009 and contains no conflict of interest statement.
Compare and contrast with an October 2009 review article (Submitted April that year) in the OA journal Open Longevity Science (which no longer exists), in which author Alan B. Cash lists a conflict of interest – namely being an officer of a company that sells OAA supplements (that would be Terra Biological, founded in 2006).
So, Dr. Cash (and the lead author on the Aging Cell paper David Williams) appear to have seen fit to disclose this rather massive COI in an OA review article, but not in an Aging Cell primary research paper. Another notable find is that Cash submitted a patent application on OAA as a CR mimetic in 2005 – a full 4 years before the paper was published. You might think such an event would require disclosure?
Back to the science, Cash’s glorious PowerPoint deck from 2014 includes claims that studies in mice were underway in 2011, in collaboration with Steve Spindler at UC Riverside. A quick PubMed search indicates that nothing was ever published on this. Similarly, claims are made about lifespan extension in flies, but again there’s nothing published.
For brain cancer, there are a couple of papers showing alleged effects of OAA in mice, but the data are just not very believable, and the dose used – 2 grams per kg (that’s not a typo) equating to 150 grams in a human – is ridiculous!
So, that leaves just about the entirety of the medical claims about OAA based on a single published paper about worms, a shady pay-to-play OA review article by the CEO of the company, and a bunch of unsubstantiated and unpublished claims in PowerPoint slides. Despite this, some people saw fit to convince the FDA to allow clinical trials – one for Parkinson’s, which failed, and another for Alzheimer’s which is ongoing (wanna bet how it’ll turn out?)
OAA cannot work the way they say it does
The claimed mechanism-of-action for OAA is that it drives the MDH enzyme reaction backwards, and this enhances levels of NAD+, which boosts the activity of the Sirtuins, which are allegedly involved in the beneficial effects of CR. There’s another whole can of worms about SIRTs and NAD+ (covered briefly here), but that’s for another day. But anyway, driving MDH backwards to make NAD+ has a number of problems…
First, by using this redox reaction as a source of NAD+, the ultimate source is of course NADH. In cells, NAD+ is normally present at about 700-fold greater levels than NADH. As such, NADH cannot possibly serve as an adequate “reservoir” to provide more NAD+. Even if you could convert all the NADH in your cells to NAD+, the effective level of NAD+ would only go up by 0.14% (and you’d probably die because – duh – no NADH left!)
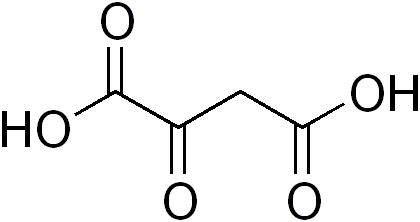
Second, OAA (above) is a dicarboxylic acid. As such, it is negatively charged at physiologic pH. Negatively charged species are generally excluded from cells. Any mitochondrial biologist can tell you the way to get dicarboxylates into cells is to block the carboxylate moieties with alkyl groups… You make methyl or ethyl esters and these get transported into cells, then the alkyl groups are cleaved off by cytosolic esterases, yielding the free dicarboxylate trapped inside the cell. This is done all the time for Krebs’ cycle metabolites, and you can buy methylated versions of succinate, alpha-ketoglutarate, etc. These molecules are proven bio-available, and the cancer drug dimethyl fumarate exploits this exact same biology to get the active agent (fumarate) into cells.
A third potential confounder is the achievable concentration in people. Remember, the worm study used a level of 8 millimolar in the media – that’s about a gram per liter (molecular weight of OAA = 132). To obtain a similar level in human plasma, assuming a typical human blood volume of 5 liters that would be 5 grams of OAA, or 50 times more than the 100 mg found in the dietary supplement being sold. It’s unlikely that 100 mg of oral OAA would appreciably increase the level of this metabolite in tissues.
Essentially, non-esterified OAA at a low external concentration will not get into cells, and unfortunately that’s where the MDH enzyme (required for the claimed mechanism-of-action) is located. It’s notable that the only evidence Alan Cash shows for an effect of OAA on NAD+/NADH levels comes from isolated mitochondrial studies in the 1960s, where of course the cell membrane is not present.
Another claimed mechanism for OAA is the lowering of blood glutamate levels. Glutamate at high levels in the brain causes excitotoxicity, and OAA is claimed to remove glutamate by converting it to a-ketglutarate, using the enzyme GOT (glutamate/oxaloacetate transaminase). Here’s the reaction:
Glutamate + OAA <–> Aspartate + a-ketoglutarate.
Except there are problems with this mechanism too. First, the enzyme GOT (there are 2 isoforms, GOT1 and GOT2) is not usually found in the plasma. In-fact, you probably know it by another name… aspartate aminotransferase, or AST. That would be the very same AST that’s used (along with ALT) as a biomarker for liver injury. AST is not supposed to be in the bloodstream – if it’s there you have liver failure!
In addition, there are several reasons to believe that the activity of the GOT enzyme is actually pro-cancer. Specifically, cancer cells often hijack the Krebs’ cycle in mitochondria to leech out carbon skeletons for building other biomolecules such as lipids and proteins. This creates a deficit in the cycle, so it’s necessary to top up the cycle – a process known as “anaplerosis”. Cancer cells use glutamate (and glutamine) as a carbon source to replenish the Krebs’ cycle; and how do they get that carbon into the cycle? Via the GOT enzyme!
Furthermore, the very paper that is used to make these claims contains data that contradicts this mechanism of action. Specifically, Figure 7 shows that glutamate levels went UP slightly with OAA treatment (not down as desired). Here’s a snapshot from the brochure for Cronaxal (from that DropBox treasure trove), explaining the GOT mechanism. It makes my brain hurt…
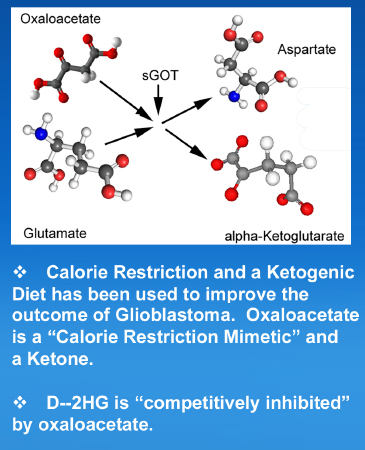
For starters, there’s the ridiculous claim that OAA is ketone. This seems disingenuously pitched to jump on the bandwagon of the ketogenic diet fad. Yes, chemically speaking OAA contains a ketone group, but it’s not a ketone body in the classical medical terms intended here. There are only 2 ketone bodies: beta-hydroxybutyrate and acetoacetate. Nothing else is a “ketone” if what you mean is something involved in ketosis, ketogenenesis, diet effects, etc.
Then there’s the claim that D-2HG is inhibited by OAA. WTF? 2HG is an “oncometabolite” made by rogue enzymes in certain forms of cancer. It is not an enzyme. Enzymes get inhibited. Metabolites are processed by enzymes. As far as I know, metabolites cannot get “inhibited” by other metabolites, but whatever, I’m just a lowly biochemist. Like I said, this makes my brain hurt.
Someone needs to go read a biochemistry text book.
The biochemistry dumb-fuckery gets even worse in this awesome slide from the Cronaxal promotional materials…
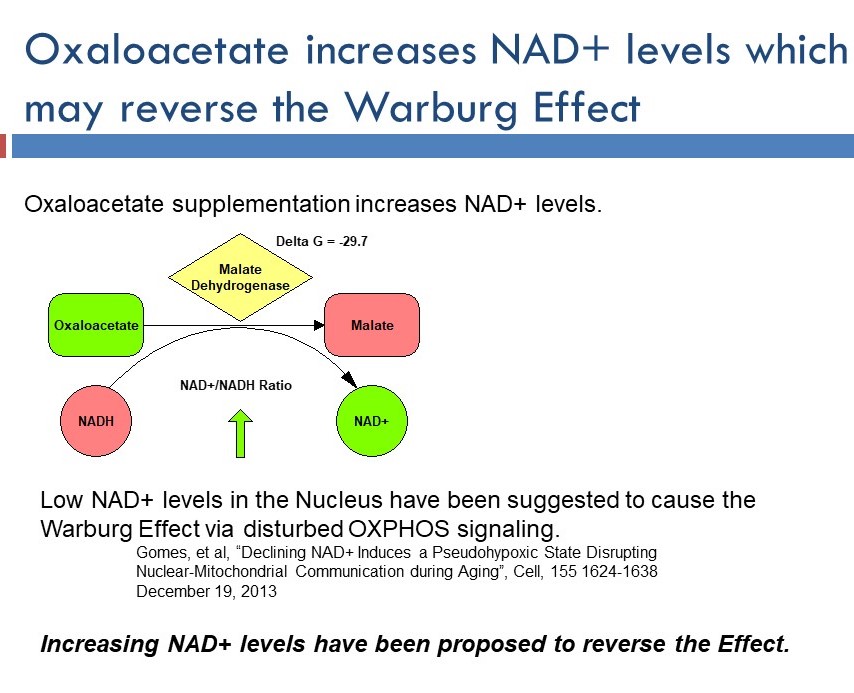
To be clear… NAD+ availability is a key determinant of the rate of glycolysis in cells. Cancer cells LOVE glycolysis (the Warburg effect). More NAD+ means more glycolysis. NAD+ will thus promote the Warburg effect. Anyone suggesting the opposite (note the lack of a reference for the statement at the bottom of the slide) is being silly.
So how might OAA be working (if it is working at all)?
In my (not so professional) opinion, there’s no freakin’ way that OAA can be having its biological effects via an MDH >> NAD+ mechanism. The biochemistry just doesn’t add up. There’s also very little chance it’s working via a GOT-dependent lowering of glutamate (and if it is, this might actually be beneficial toward cancer).
So, how could it be working? There are a number of known G-protein coupled receptors for Krebs’ cycle metabolites expressed on the surface of cells – most notably the succinate receptor GPR91. Whether such receptors can respond to OAA in the plasma is not known. Another interesting property of OAA (and indeed all alpha-keto acids) is that it reacts directly with hydrogen peroxide. Mixing OAA with H2O2 gets you malonate, another metabolite with biological effects. It has been claimed that OAA is an antioxidant, but that’sa bit of a stretch. A third interesting property of OAA… it’s a potent inhibitor of mitochondrial respiratory complex II (succinate dehydrogenase), but of course such an effect would require it to get across cell membranes, oops!
Conclusion
So there you have it, a molecule that doesn’t get into cells, with no published primary scientific data in mammals indicating an effect at an achievable dose, and has failed in at least one clinical trial, hyped to hell by a zealous CEO who doesn’t disclose COIs when publishing, mostly based on a single paper in worms, now being pushed on desperate glioma patients, with a complete lack of understanding of the fundamentals of biochemistry, all wrapped up in a bunch of slick brochures and at least 5 different supplement companies all run by one person. What a shit-show!
